Introduction:
The misfolding and clumping of proteins, termed aggregation, are hallmarks of several neurodegenerative diseases including Alzheimer's disease, Parkinson's disease, and Huntington's disease. These aggregated proteins disrupt cellular processes, leading to nerve cell dysfunction and death. Effective therapies for these diseases rely on our ability to disarm these toxic proteins by preventing their formation or eliminating them after they form. This blog post will explore current research on therapeutic strategies targeting protein aggregation.
Targeting Misfolded Proteins:
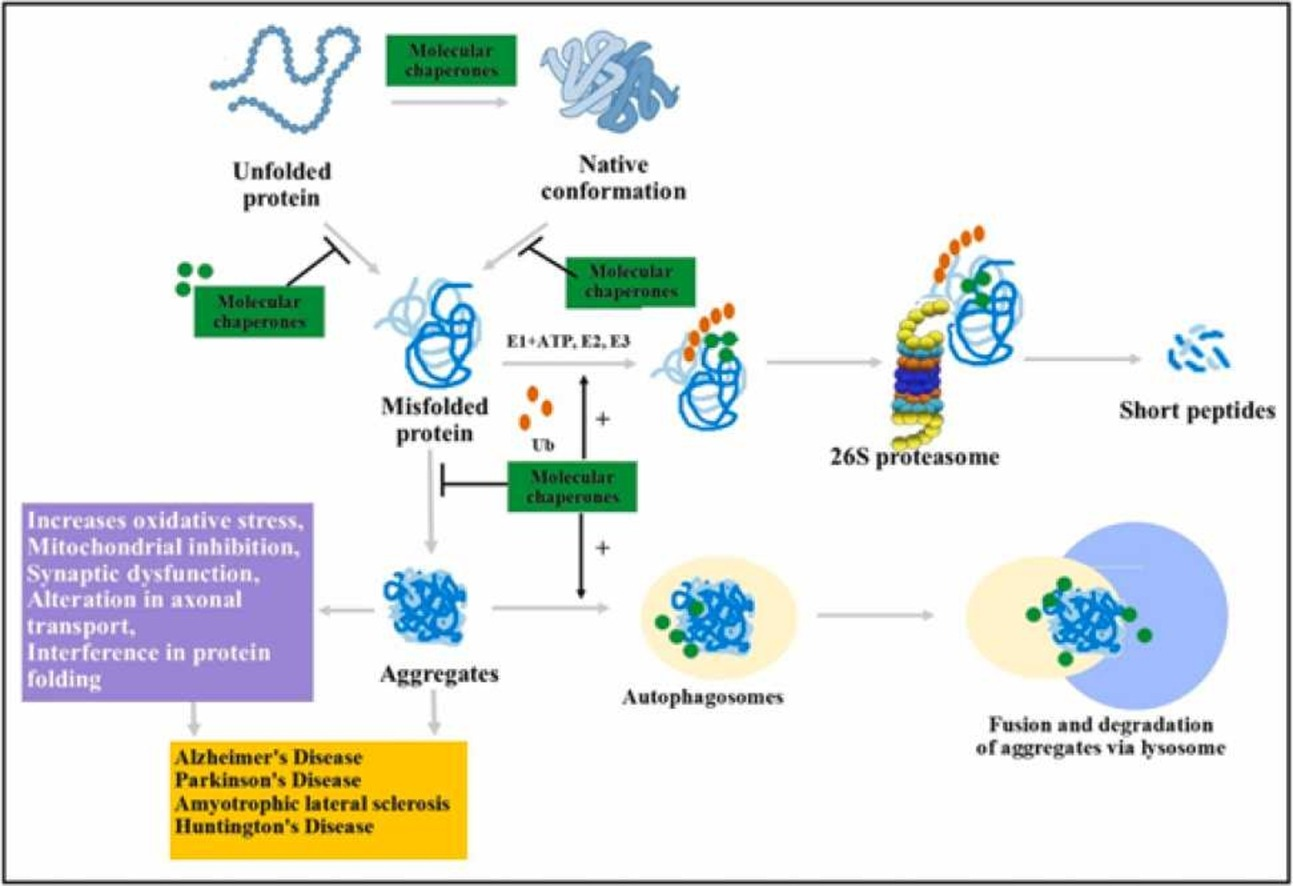
One approach involves molecular chaperones, proteins that assist other proteins in folding correctly. Small molecule chaperones mimic the function of natural chaperones, binding to misfolded proteins and refolding them into functional forms. Verubecestat, a medication approved by the FDA for Alzheimer's disease, exemplifies this strategy. It stabilizes beta-amyloid precursor protein (AβPP), thereby reducing the formation of amyloid plaques.
Enhancing Protein Clearance:
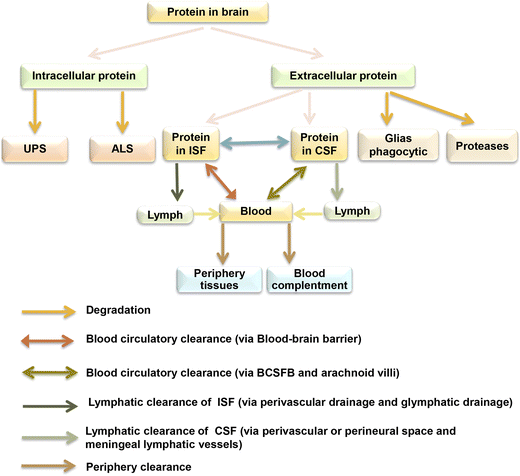
Another strategy leverages the body's natural protein degradation system, the ubiquitin-proteasome system (UPS), and autophagy pathway. The UPS targets proteins for breakdown by attaching ubiquitin molecules to them. Researchers are developing methods to improve the UPS's recognition and clearance of misfolded proteins, such as creating small molecule modulators for specific E3 ubiquitin ligases. Autophagy, a cellular recycling process, can also engulf and break down protein aggregates. Activating autophagy through pharmacological agents holds promise as a therapeutic strategy, although challenges related to specificity and potential side effects remain.
Preventing Aggregation of Misfolded Proteins:
Anti-amyloidogenic compounds directly target misfolded proteins to prevent them from clumping together. These molecules bind to specific shapes of the protein, hindering their ability to self-assemble. For instance, certain antibodies have shown effectiveness in reducing amyloid plaque burden in preclinical models.
Challenges and Future Directions:
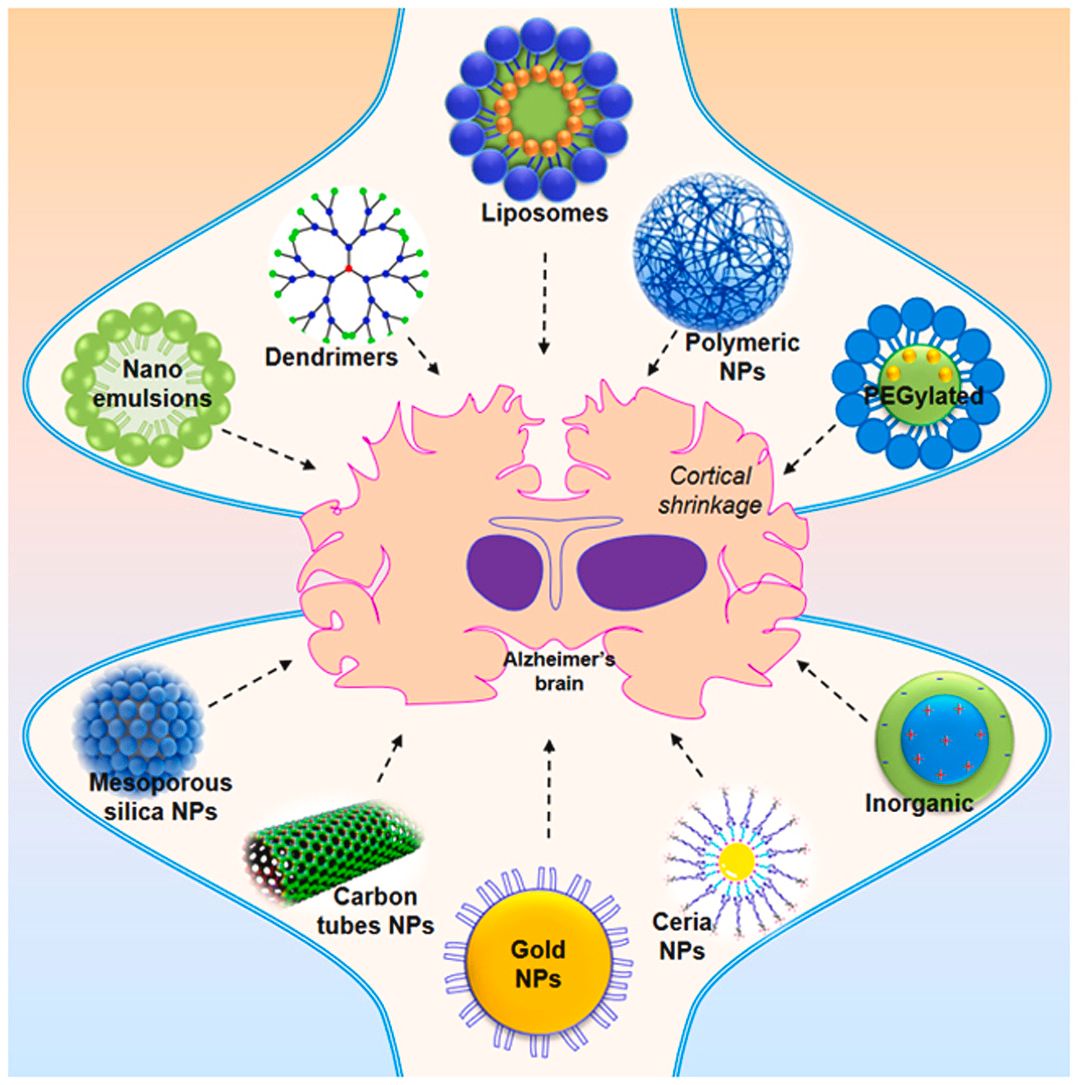
Despite significant progress, challenges remain in developing effective anti-aggregation therapies. The blood-brain barrier limits the delivery of many drugs to the central nervous system. Additionally, the variety of protein aggregates across different neurodegenerative diseases necessitates targeted therapies specific to each condition.
Future directions include utilizing advanced computational modeling to design more potent and selective anti-amyloidogenic compounds. Moreover, research on combination therapies targeting different aspects of the aggregation process, alongside exploring gene editing techniques to address the underlying genetic causes of protein misfolding, hold promise for a more comprehensive therapeutic approach.
Importance of High-Quality Research Reagents:
Success in this field relies on access to high-quality research reagents, including purified proteins, antibodies, and biochemicals. Companies like Gentaur Group, a leading supplier of life science research products, play a vital role by providing scientists with the tools they need to advance our understanding of proteinopathies and develop effective therapies.
Conclusion:
Protein aggregation is a critical area of research in the fight against neurodegenerative diseases. By targeting misfolded proteins, enhancing clearance mechanisms, and preventing aggregation itself, researchers are developing a diverse range of therapeutic strategies. Overcoming the current challenges and capitalizing on emerging technologies offer hope for a future where we can effectively eliminate these toxic proteins and prevent the devastating consequences of protein aggregation.
Learn more by watching the video below