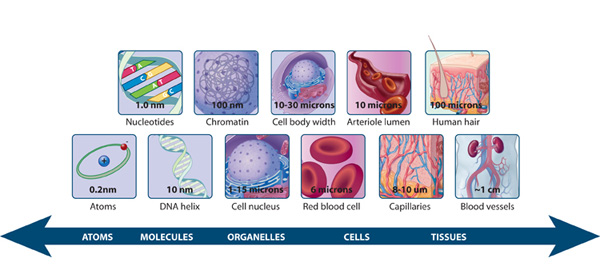
Introduction
Cellular functions are orchestrated by a complex interplay of organelles and macromolecules. To fully understand these processes, researchers require the ability to observe them at high resolution. The invention of the microscope in the 17th century revolutionized biological exploration. However, conventional optical microscopes are hampered by the diffraction barrier, which restricts the visualization of structures smaller than half the wavelength of light used. This has limited our ability to delve into the intricate organization and dynamics of cellular structures at the nanoscale.
Super-Resolution Microscopy: Transcending the Diffraction Barrier
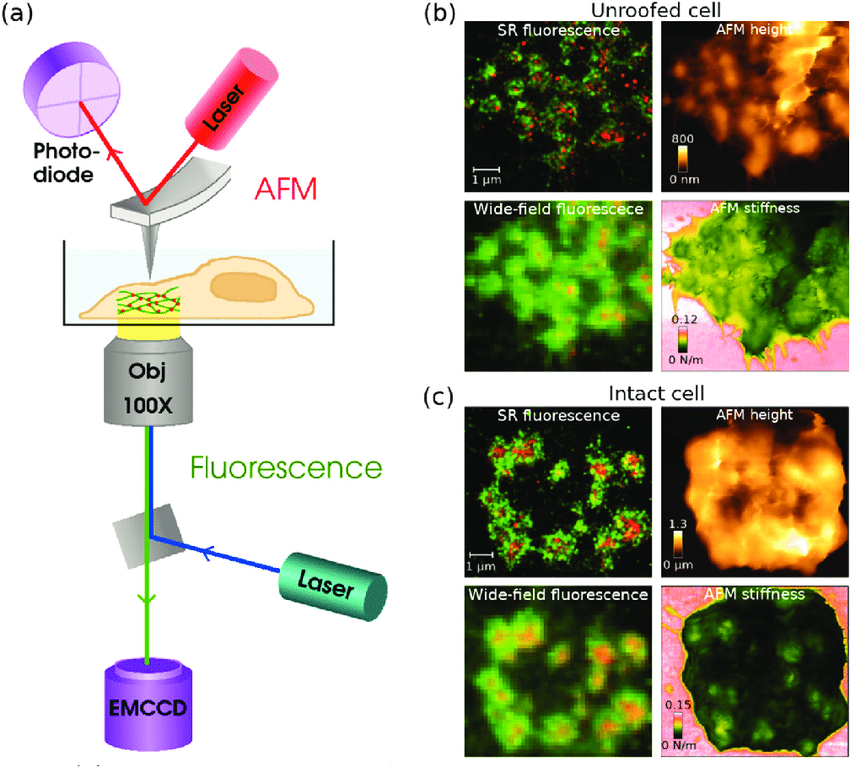
Super-resolution microscopy techniques have emerged as a transformative force in cellular imaging, overcoming the diffraction barrier. These techniques exploit various physical principles to achieve spatial resolutions down to tens of nanometers. Here, we explore three prominent super-resolution microscopy methods:
- Single-Molecule Localization Microscopy (SMLM): SMLM encompasses techniques like Stochastic Optical Reconstruction Microscopy (STORM) and Photoactivated Localization Microscopy (PALM). These methods rely on the stochastic activation and precise localization of individual fluorophores within a sample. By statistically reconstructing the positions of numerous fluorophore activations, SMLM generates high-resolution images with exceptional detail.
- Stimulated Emission Depletion (STED) Microscopy: STED utilizes a sophisticated illumination strategy. A doughnut-shaped depletion beam co-localized with an excitation beam suppresses fluorescence in designated regions of the sample. This effectively confines the excited fluorophore population to a smaller area, leading to enhanced resolution compared to conventional fluorescence microscopy.
- Structured Illumination Microscopy (SIM): SIM projects a patterned illumination grid onto the sample to convey high-frequency information. By acquiring multiple images with varying grid rotations and computationally deconvolving them, SIM achieves improved resolution compared to wide-field microscopy.
Impact of Super-Resolution Microscopy on Cellular Research
Super-resolution microscopy has profoundly impacted various domains of biological research:
- Deciphering Cellular Architecture: These techniques enable high-resolution visualization of intricate protein-protein interactions within organelles, elucidate the nanoscale organization of the cytoskeleton, and reveal the dynamics of macromolecular assemblies. For instance, STORM microscopy has been instrumental in elucidating the architecture of the immunological synapse, a specialized interface between immune cells and their targets.
- Investigating Cellular Dynamics: High temporal resolution imaging facilitates real-time observation of biological processes. This capability has advanced the study of phenomena such as protein trafficking, changes in organelle morphology, and macromolecular interactions within living cells.
- 3D Visualization of Cells: Super-resolution techniques extend beyond two-dimensional imaging. By acquiring multiple images at different focal depths and computationally deconvolving them, researchers can generate high-resolution 3D reconstructions of entire cells. This provides a comprehensive view of cellular organization, enhancing our understanding of cellular functions.
Challenges and Future Directions
Despite their advanced capabilities, super-resolution microscopy techniques have limitations. They often require specialized fluorophores and complex instrumentation, limiting their accessibility. Additionally, the extended acquisition times for super-resolution images can be a barrier for studying highly dynamic biological processes.
Ongoing advancements are expected to mitigate these limitations. Developments in novel fluorophores with improved photophysical properties, enhancements in instrumentation for more efficient image acquisition, and automation for high-throughput analysis are promising areas of research.
The following video explains super-resolution microscopy principles in more detail.
![Organisation and cellular dynamics of the comb. (A) Scanning electron microscopy view of two successive combs along a comb row. The dotted line materialises the outline of the comb basal cushion (bc), the cilia (ci) emerging along its midline. (B) Schematic transverse section of a basal cushion according to the double-arrowed line in (A) (after [70]). Polster cell bodies are represented in white, the covering epithelium in Organisation and cellular dynamics of the comb. (A) Scanning electron microscopy view of two successive combs along a comb row. The dotted line materialises the outline of the comb basal cushion (bc), the cilia (ci) emerging along its midline. (B) Schematic transverse section of a basal cushion according to the double-arrowed line in (A) (after [70]). Polster cell bodies are represented in white, the covering epithelium in](/web/image/41473-69a75ffb/image.png?access_token=52007197-69b7-4ee9-9c1d-eff554914a30)