Polymerase Chain Reaction (PCR) is a foundation of molecular biology, allowing scientists to amplify specific DNA sequences. Since its invention in the 1980s, PCR has seen significant advancements. Two prevalent forms are Traditional PCR (endpoint PCR) and Real-Time PCR (quantitative PCR or qPCR). This study directly compares their core principles, advantages, and applications.
Basic Principles:
Traditional PCR:
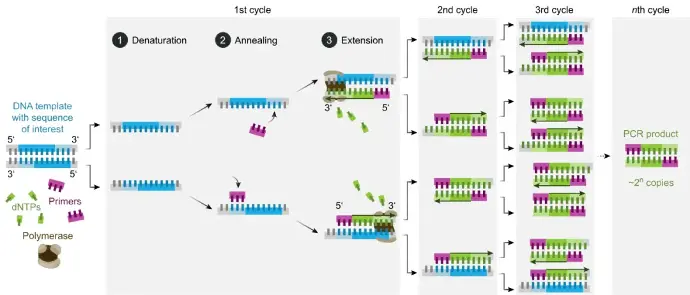
Traditional PCR involves repeated cycles of three steps:
- Denaturation: High temperature separates the double-stranded DNA into single strands.
- Annealing: Primers, short DNA sequences complementary to the target region, bind to each single strand.
- Extension: Taq polymerase, a heat-resistant enzyme, synthesizes new DNA strands complementary to the target sequence using the primers as starting points.
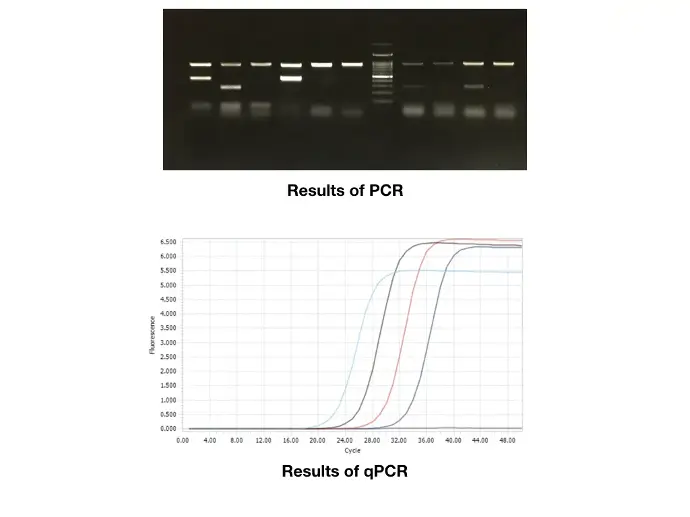
These cycles are repeated, exponentially amplifying the target DNA fragment. Analysis of the amplified products (amplicons) typically occurs at the end using gel electrophoresis. This method provides qualitative or semi-quantitative data about the DNA.
Real-Time PCR:
Real-Time PCR also follows the cyclical process but incorporates a fluorescent dye or probe to measure the amount of DNA after each cycle. This allows for quantification of DNA during the exponential phase, enabling precise and quantitative analysis.
Enzymes in PCR:
Both methods rely on Taq polymerase, a heat-stable enzyme that catalyzes the extension of DNA strands during the PCR process.
Sensitivity and Quantification:
Traditional PCR:
Sensitivity: Traditional PCR is highly sensitive, detecting minute amounts of DNA. However, it primarily offers qualitative data.
Quantification: Quantification is indirect and less accurate, relying on the intensity of bands in gel electrophoresis compared to a standard curve.
Real-Time PCR:
Sensitivity: Real-Time PCR enhances sensitivity by detecting PCR product accumulation in real-time, often leading to higher precision for low-abundance targets.
Quantification: Real-Time PCR offers direct and accurate quantification of DNA. Fluorescence intensity correlates with the amount of DNA, providing quantitative data over a wider range.
Speed and Throughput:
Traditional PCR:
Speed: Traditional PCR is generally slower because it requires additional post-amplification steps for analysis, like gel electrophoresis and staining.
Throughput: Lower due to these extra steps and the time-consuming nature of gel electrophoresis.
Real-Time PCR:
Speed: Real-Time PCR is faster as detection and quantification occur simultaneously with amplification, eliminating the need for post-PCR analysis.
Throughput: Higher throughput is achievable due to automation and the ability to process multiple samples concurrently.
Specificity and Versatility:
Traditional PCR:
Specificity: Traditional PCR can achieve high specificity with proper primer design, but may require optimization to reduce non-specific amplification.
Versatility: It is versatile for various applications, including cloning, sequencing, and genotyping, but lacks real-time analysis capabilities.
Real-Time PCR:
Specificity: Real-Time PCR enhances specificity through sequence-specific probes (like TaqMan probes) that reduce non-specific signals.
Versatility: In addition to traditional applications, Real-Time PCR is particularly suited for quantitative studies like gene expression analysis, pathogen detection, and high-specificity genotyping.
Applications:
Traditional PCR:
- Commonly used in research labs for cloning, sequencing, mutagenesis, and genotyping.
- Suitable for applications where quantification is not critical.
Real-Time PCR:
- Widely used in clinical diagnostics for detecting and quantifying pathogens like viruses and bacteria.
- Essential in research for gene expression analysis, quantifying genetic variations, and validating microarray data.
- Applied in forensics and food safety for identifying and quantifying specific DNA sequences in complex mixtures.
Conclusion:
Both Traditional PCR and Real-Time PCR are powerful tools in molecular biology, each with distinct advantages. Traditional PCR is cost-effective and versatile for various qualitative applications. Real-Time PCR offers superior sensitivity, specificity, and quantification capabilities, making it ideal for quantitative analyses and high-throughput diagnostics. The choice between these techniques depends on the specific needs of the experiment, including the need for quantification, speed, and throughput. As molecular biology advances, Real-Time PCR is likely to become even more integral to research and diagnostics, while Traditional PCR will remain a valuable tool for fundamental applications.