Introduction
Analytical chemistry is essential for scientific research and technological development. It allows scientists to identify, measure, and understand the chemical makeup of materials. Reagents are the chemicals used in these techniques and play a critical role in accurate analysis. They react specifically with target substances (analytes) to reveal information about the sample's composition. This blog post explores the different types of reagents used in analytical chemistry and provides tips for selecting high-quality suppliers like Maxanim Ltd to ensure accurate and reliable results.
Essential Reagents for Analytical Techniques
There are many different types of reagents, each suited for specific analytical methods. Here are some key examples:
- Gravimetric Analysis: This technique involves precipitating the analyte as a solid compound. Common precipitating agents include barium chloride (BaCl₂) for detecting sulfate (SO₄²⁻) and silver nitrate (AgNO₃) for analyzing chloride (Cl⁻).
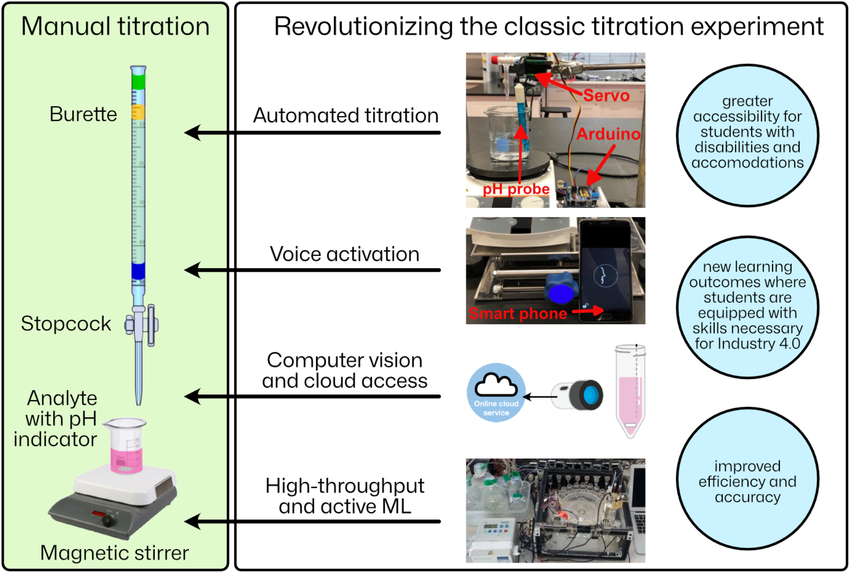
- Volumetric Analysis: In titrations, a standardized solution (titrant) reacts with the analyte until an endpoint is reached. This endpoint is often indicated by a color change using an indicator. Examples of titrants include sodium hydroxide (NaOH) for acid-base titrations and potassium permanganate (KMnO₄) for redox titrations.
- Spectrophotometry: This technique measures how light interacts with the sample. Chromogenic reagents, like those used in Bradford assays for protein measurement, react with the analyte to produce colored products with unique light absorption patterns. Complexing agents, such as ethylenediaminetetraacetic acid (EDTA), can bind to metal ions, affecting their light-absorbing properties.
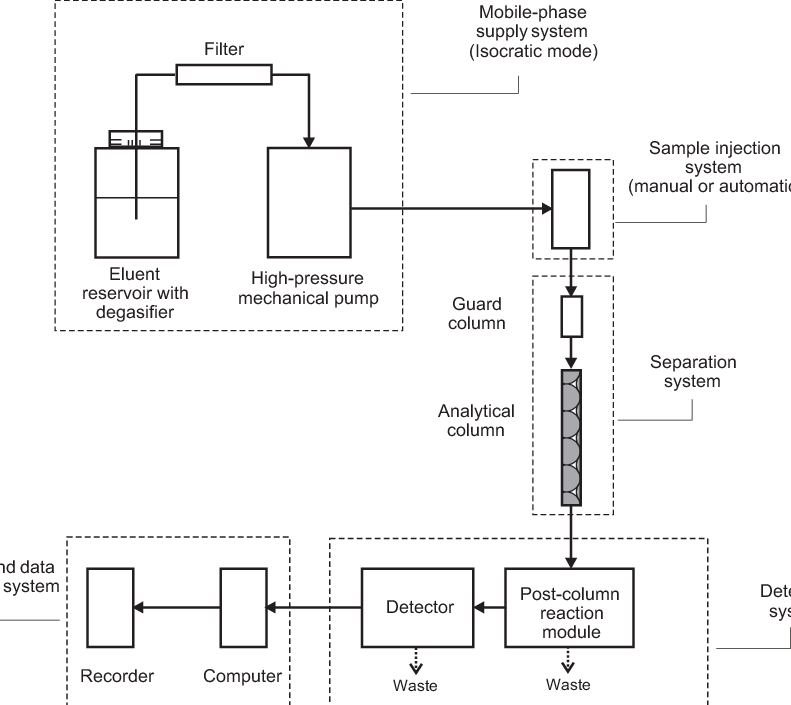
- Chromatography: Separation techniques like high-performance liquid chromatography (HPLC) use mobile phases (solvents) and stationary phases (columns) to separate analytes based on their interactions with these components. Buffer solutions maintain a consistent pH, which is crucial for optimal separation.
Selecting High-Quality Reagents
The quality of analytical data depends on choosing the right reagents and using them properly. Here are some tips for selecting reliable reagent suppliers:

- Reagent Purity: Look for analytical grade reagents whenever possible. Impurities can interfere with the analysis and lead to inaccurate results.
- Standardization: Regularly standardize titrants using primary standards (compounds with known high purity) to ensure accurate concentration determination.
- Storage Conditions: Follow recommended storage conditions for reagents to maintain their effectiveness. Temperature, light exposure, and moisture can all affect reagent activity.
- Calibration: Regularly calibrate instruments using appropriate standards to ensure accurate measurements.
Conclusion
Reagents are essential tools for analytical chemists. By understanding the different types of reagents and how to choose high-quality suppliers, researchers can ensure accurate and reliable data, ultimately supporting scientific progress.