Brain regeneration, the process by which damaged neurons are replaced and neural circuits are restored, is a vital area of research for neurological disorders and injuries. While the adult mammalian brain has limited regenerative capacity compared to other animals, scientists are actively investigating the molecular pathways involved. Understanding these pathways is essential for developing treatments to promote brain repair after stroke, traumatic brain injury, and neurodegenerative diseases.
Neurogenesis and Neural Stem Cells (NSCs):
At the center of brain regeneration lies the activation of NSCs, found in specific areas like the subventricular zone (SVZ) and the subgranular zone (SGZ) of the hippocampus. These cells can both self-renew and differentiate into new neurons. The Notch signaling pathway regulates NSC fate. Notch activation promotes self-renewal, while Sonic hedgehog (Shh) pathway activation promotes differentiation into mature neurons.
Growth Factor Signaling:
Multiple growth factors play a role in the neurogenic response following injury. Brain-derived neurotrophic factor (BDNF) stimulates NSC proliferation, survival, and neurite outgrowth. Fibroblast growth factor (FGF) signaling also enhances NSC proliferation and differentiation. These growth factors activate signaling cascades that regulate various aspects of neurogenesis.
Maxanim (Gentaur Group) offers Acidic Fibroblast Growth Factor (FGF 1) human (Recombinant) for researchers interested in studying its role in neurogenesis and other cellular processes (for more information you can visit our website).
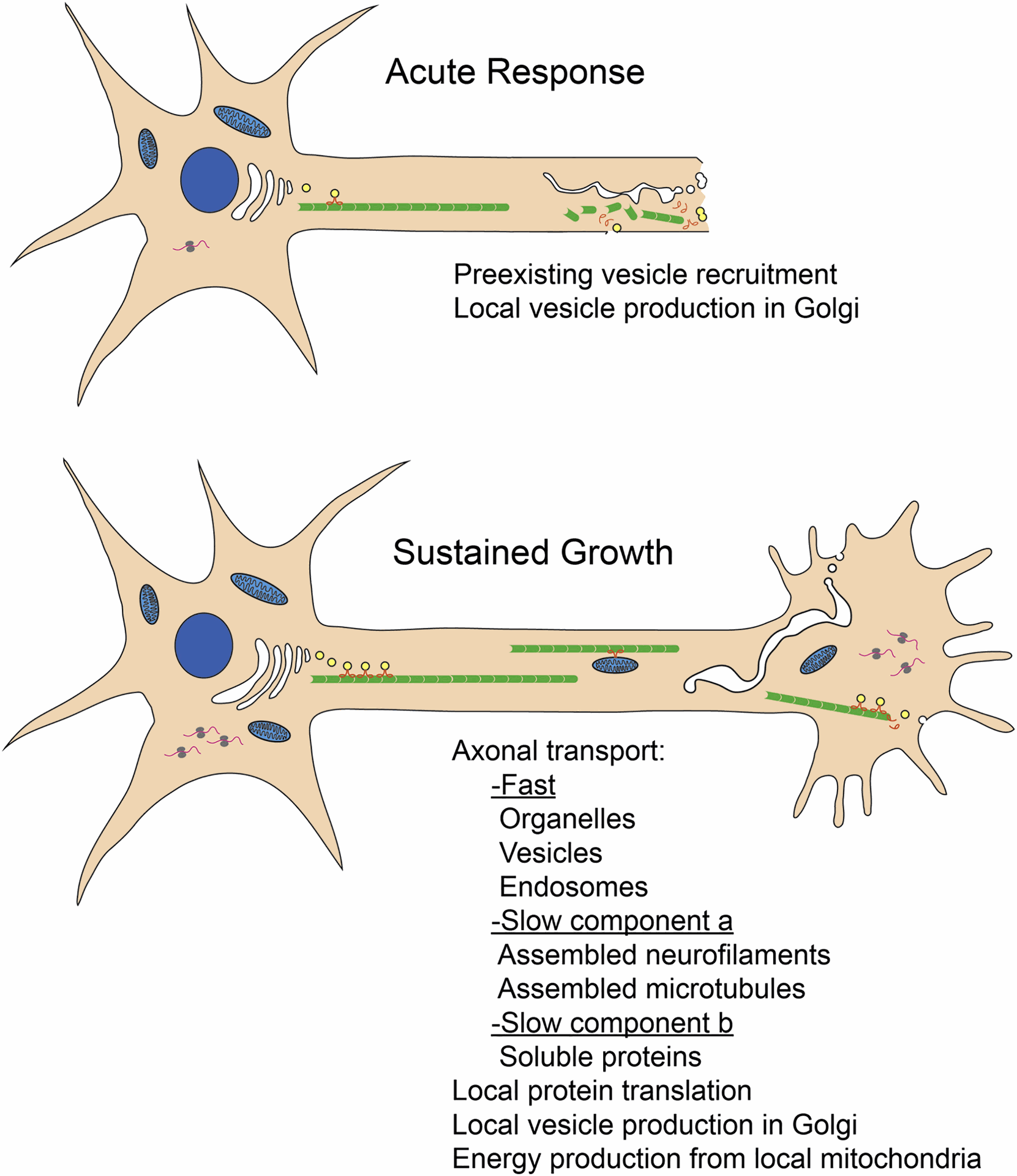
Regulation of Axonal Regeneration:
After injury, severed axons undergo a complex process of regeneration, involving growth cone extension and re-establishment of synaptic connections. The Wnt signaling pathway is a critical driver of axonal regeneration by promoting the expression of genes essential for growth cone formation and elongation. The mTOR pathway has a complex role, with initial activation promoting axon outgrowth but prolonged activation hindering regeneration.
Inflammatory Response and Neuroprotection:
Inflammation is a double-edged sword in brain regeneration. The initial inflammatory response after injury clears debris and promotes repair, but chronic inflammation can be detrimental. Microglia, the resident immune cells of the brain, play a crucial role in orchestrating this response. Neurotrophic factors like insulin-like growth factor 1 (IGF-1) and cytokines like interleukin-10 (IL-10) promote a pro-regenerative microenvironment.
Epigenetic Regulation:
Epigenetic modifications, such as DNA methylation and histone acetylation, play a critical role in regulating gene expression and cellular fate. Following injury, the epigenetic landscape of NSCs and surrounding cells is dynamically remodeled to promote a regenerative program.
Future Directions:
Understanding these molecular pathways paves the way for developing novel therapeutic strategies. Pharmacological approaches aimed at modulating specific signaling cascades hold promise for promoting neurogenesis, enhancing axonal regeneration, and creating a regenerative microenvironment after brain injury.
Explore peripheral nerve regeneration on a cellular level (video)
![Stem cell niche and neurogenesis in adult. Neural stem cells (NSCs) are mostly retained in two regions: Sub-ventricular zone (SVZ) and sub-granular zone (SGZ). (A) Frontal cross-section of the adult brain showing the location of the SVZ, in walls of the lateral ventricles (V). In the SVZ, NSCs correspond to type B1 cells. B1 cells express Cx26, Cx43 and Cx45. While Cx43 expression negatively regulates cell proliferation, Cx45 exhibits an opposite role. These B1 cells generate intermediate progenitor cells (IPCs) corresponding to C cells that divide to generate neuroblasts (type A cells). (B) Frontal cross-section of the adult brain showing the hippocampal formation. The insert indicates the location of the dentate gyrus. The adult dentate gyrus contains radial glial cells, which are polarized cells with their cell body in the SGZ. Radial glial cells (RGC or RC, type 1 cells) generate IPCs (IPC1 and IPC2 or type 2a and type 2b cells), which differentiate into immature granule cells (IGCs or type 3 cells) and mature granule cells (GC). In SGZ, Cx26, Cx30, Cx36, Cx37, Cx40, Cx43, Cx45 are expressed in various NPCs. Interestingly, Cx30 and Cx43 seem to be active regulators of hippocampal NPC proliferation while Cx43 itself is a negative regulator of proliferation in SVZ [258]. Stem cell niche and neurogenesis in adult. Neural stem cells (NSCs) are mostly retained in two regions: Sub-ventricular zone (SVZ) and sub-granular zone (SGZ). (A) Frontal cross-section of the adult brain showing the location of the SVZ, in walls of the lateral ventricles (V). In the SVZ, NSCs correspond to type B1 cells. B1 cells express Cx26, Cx43 and Cx45. While Cx43 expression negatively regulates cell proliferation, Cx45 exhibits an opposite role. These B1 cells generate intermediate progenitor cells (IPCs) corresponding to C cells that divide to generate neuroblasts (type A cells). (B) Frontal cross-section of the adult brain showing the hippocampal formation. The insert indicates the location of the dentate gyrus. The adult dentate gyrus contains radial glial cells, which are polarized cells with their cell body in the SGZ. Radial glial cells (RGC or RC, type 1 cells) generate IPCs (IPC1 and IPC2 or type 2a and type 2b cells), which differentiate into immature granule cells (IGCs or type 3 cells) and mature granule cells (GC). In SGZ, Cx26, Cx30, Cx36, Cx37, Cx40, Cx43, Cx45 are expressed in various NPCs. Interestingly, Cx30 and Cx43 seem to be active regulators of hippocampal NPC proliferation while Cx43 itself is a negative regulator of proliferation in SVZ [258].](/web/image/41987-9d5b3630/image.png?access_token=0a9a44be-c05a-45d1-b730-d41115d67eaf)