Introduction
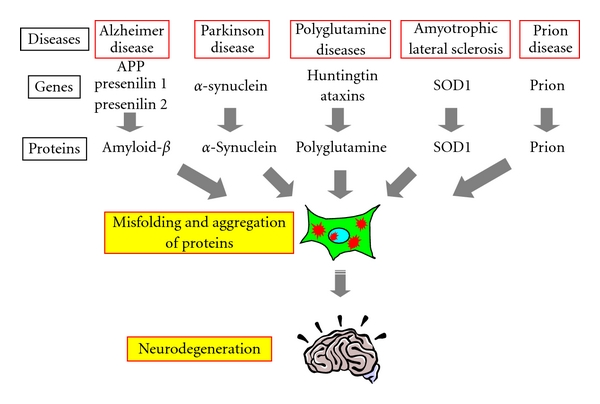
Proteins are essential molecules in cells with a wide range of functions. Their proper function depends on their three-dimensional structure, which is achieved through a specific folding process. In some cases, proteins fold incorrectly, resulting in abnormal aggregates called amyloids. These amyloids are increasingly recognized as a major contributor to neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS). This blog post examines how misfolded proteins can cause damage to neurons.
Protein Misfolding and Aggregation
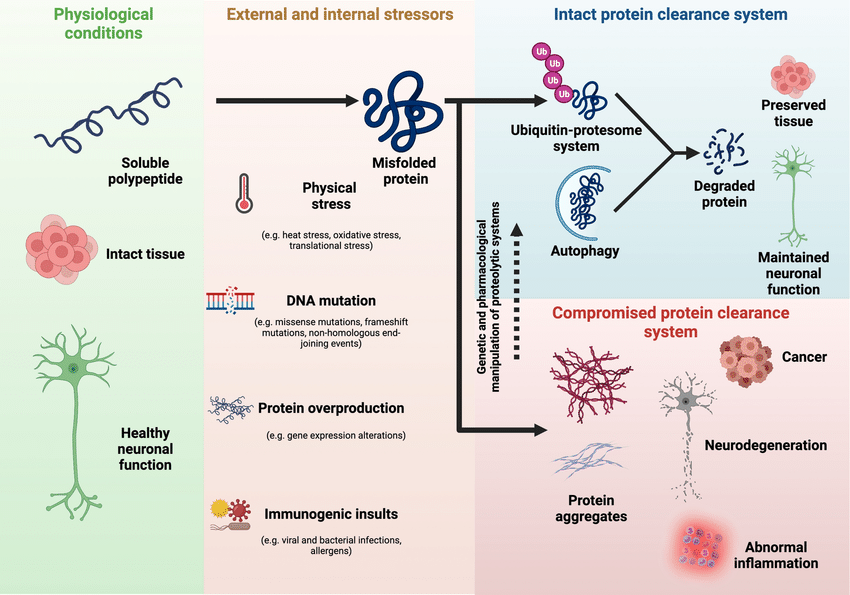
Protein folding is a complex process guided by the amino acid sequence of each protein. Molecular chaperones and cellular quality control mechanisms are crucial for ensuring proper folding. However, genetic mutations, cellular stress, and aging can disrupt this process, leading to protein misfolding. Misfolded proteins lose their intended function and often clump together, forming insoluble oligomers and fibrils. These aggregates are the characteristic pathological lesions observed in many neurodegenerative diseases.
Mechanisms of Neuronal Damage by Misfolded Proteins
The exact mechanisms by which misfolded protein aggregates harm neurons are still being investigated. However, several key pathways have been identified:
- Disrupted Proteostasis: The buildup of misfolded proteins overwhelms the cellular clearance mechanisms, leading to a toxic accumulation within neurons. This disrupts cellular proteostasis, the delicate balance between protein synthesis, folding, and degradation.
- Membrane Dysfunction: Misfolded protein aggregates can disrupt the structure and function of neuronal membranes, hindering essential cellular processes like ion transport and neurotransmitter signaling.
- Prion-like Propagation: In some neurodegenerative diseases, misfolded proteins can behave similarly to prions. These "seeds" can trigger the misfolding of healthy proteins, leading to a self-perpetuating cascade of aggregation that amplifies neurotoxicity.
- Neuroinflammation: The presence of misfolded protein aggregates can trigger an inflammatory response in the brain. This glial activation, although initially intended to be protective, can ultimately contribute to neuronal damage.
Specific Examples: Misfolded Proteins in Neurodegenerative Diseases
- Alzheimer's Disease: In AD, the aggregation of amyloid-beta (Aβ) peptides and tau protein are the defining neuropathological hallmarks. Aβ plaques and neurofibrillary tangles, composed of these misfolded proteins, are believed to disrupt neuronal function and communication. Researchers studying AD are increasingly interested in understanding the initial steps of protein misfolding and aggregation to identify potential therapeutic targets. Companies like Gentaur Group, a leading supplier of research reagents, provide essential tools for researchers to study these processes at the molecular level.
- Parkinson's Disease: PD is characterized by the accumulation of α-synuclein protein aggregates, forming Lewy bodies within dopaminergic neurons in the substantia nigra. These aggregates are thought to impair dopamine production and disrupt neuronal circuitry, leading to the characteristic motor symptoms of PD.
Conclusion
Misfolded proteins are not merely bystanders; they actively contribute to the development of neurodegenerative diseases. Understanding how these misfolded proteins cause neuronal dysfunction and death is essential for developing effective treatments. Current research is exploring strategies to target protein misfolding and aggregation, with the goal of one day halting or reversing the progression of these debilitating diseases.
Learn more about Misfolded Proteins: The Core Problem in Neurodegenerative Disease