Traditionally, immunology and metabolism were considered separate areas of study. However, the emerging field of immunometabolism reveals a critical link between these two fundamental biological processes. This article explores how cellular metabolic pathways influence immune cell behavior and how targeting these pathways may hold promise for future therapies.
Metabolic Shifts During Immune Responses:
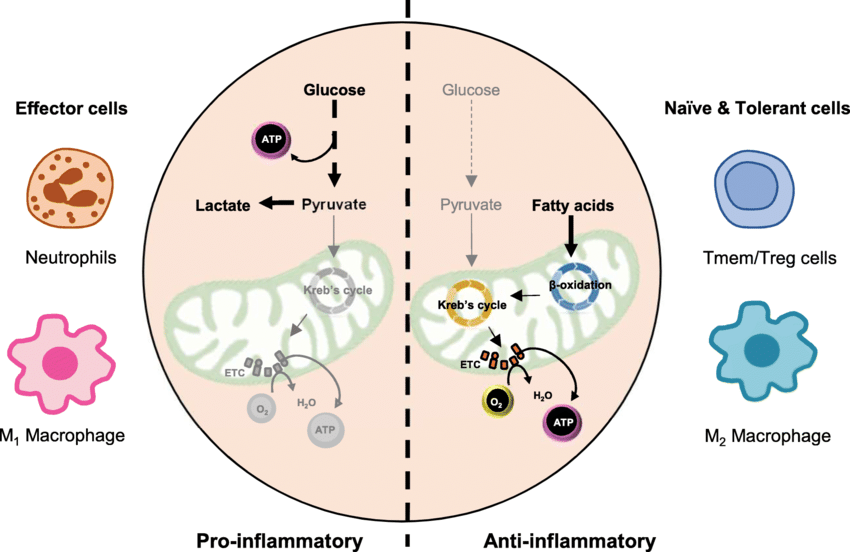
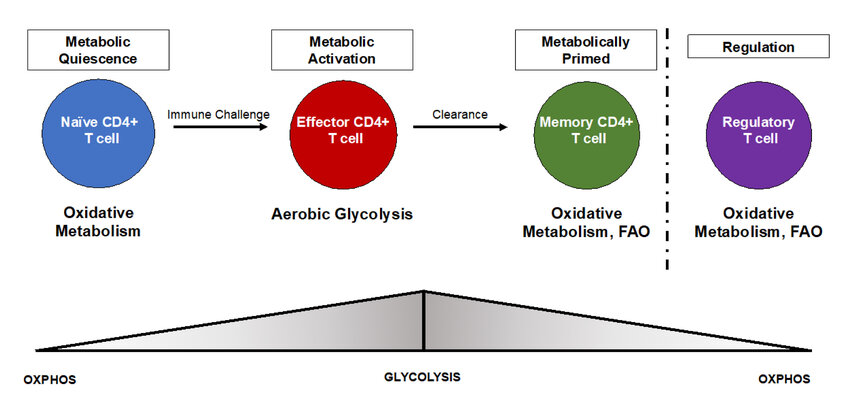
Immune cells activated to fight infection or disease undergo a significant metabolic change. Resting immune cells primarily rely on oxidative phosphorylation for energy production, but activated cells switch to aerobic glycolysis, even in oxygen-rich environments. This phenomenon, known as the Warburg effect, allows activated immune cells to rapidly generate energy to support functions like proliferation, cytokine production, and cell killing. Key metabolic pathways involved in immunometabolism include:
- Glucose metabolism: Activated immune cells take up more glucose and convert it to lactate through aerobic glycolysis, providing a quick source of energy.
- Glutaminolysis: Glutamine is a vital building block for nucleotides, amino acids, and energy production through the tricarboxylic acid (TCA) cycle.
- Fatty acid metabolism: Fatty acid synthesis and breakdown play essential roles in immune cell differentiation and function.
Metabolic Influence on Immune Cell Fate:
Metabolic cues not only fuel immune responses but also significantly impact the fate and function of immune cells. For example, T cell activation and differentiation are closely linked to specific metabolic programs. Th1 cells, which promote cellular immunity, exhibit increased glycolysis and glutaminolysis, while Th17 cells, involved in inflammatory responses, rely more heavily on fatty acid metabolism. Understanding these metabolic dependencies offers potential strategies for manipulating immune cell responses. Companies like Maxanim, a supplier of research reagents, contribute to this field by providing tools to study these intricate metabolic pathways.
Therapeutic Targeting of Immunometabolism:
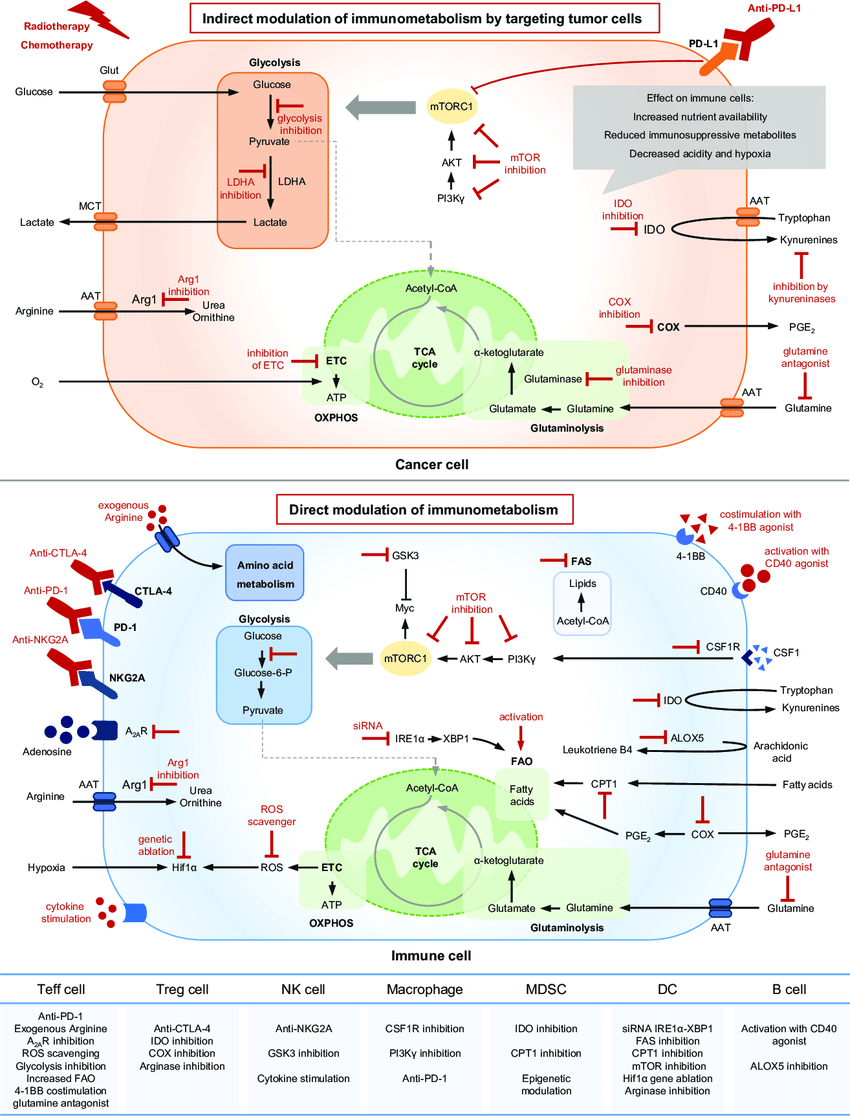
The recognition of immunometabolism's role in disease development has opened doors for novel therapeutic approaches. By targeting specific metabolic pathways in immune cells, researchers aim to modulate immune function in various disease contexts:
- Autoimmunity: Inhibiting glycolysis in T cells has shown promise in reducing autoimmune responses.
- Cancer: Reprogramming tumor metabolism or targeting the metabolic connection between tumors and immune cells holds therapeutic potential.
- Infectious Diseases: Manipulating the metabolic environment can enhance immune responses against pathogens.
Future Directions in Immunometabolism Research:
Immunometabolism is a rapidly evolving field with the potential to revolutionize our understanding of immune function and disease development. Future research efforts will likely focus on:
- Deciphering the complex signaling networks connecting metabolism and immune cell signaling pathways.
- Identifying new immunometabolic targets for therapeutic intervention.
- Developing personalized immunometabolic therapies tailored to specific disease contexts.
Conclusion:
Immunometabolism offers a powerful new lens for understanding the immune system. By elucidating the intricate link between metabolic pathways and immune cell function, researchers are paving the way for a new generation of therapeutic strategies to combat a wide range of human diseases.