Cancer immunotherapy has revolutionized cancer treatment by harnessing the immune system to fight tumors. However, some patients show limited response, necessitating exploration of new therapeutic approaches. Epigenetic modifications, alterations in gene expression without DNA sequence changes, have emerged as a promising area for overcoming these limitations. This article explores how epigenetic modifications influence cancer immunotherapy and their potential to improve patient outcomes.
Epigenetic in Cancer and Immunotherapy
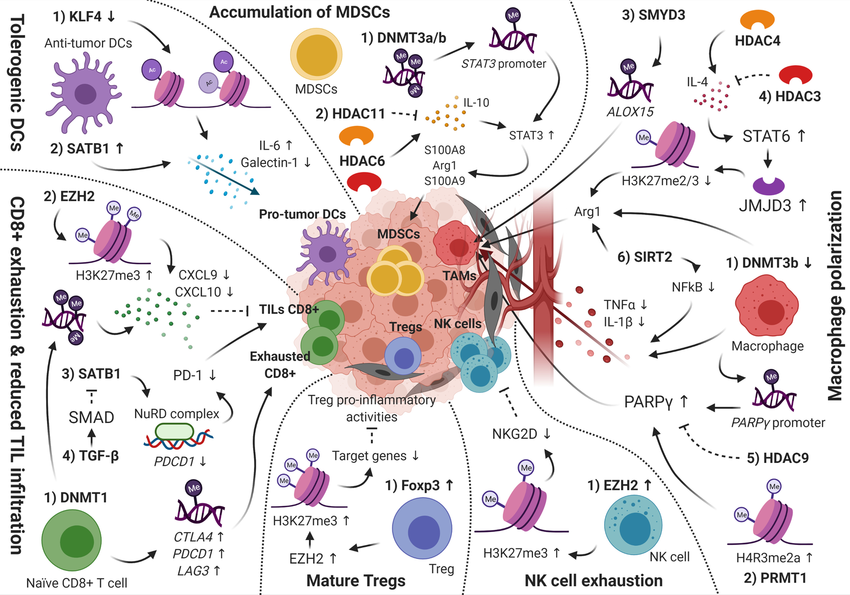
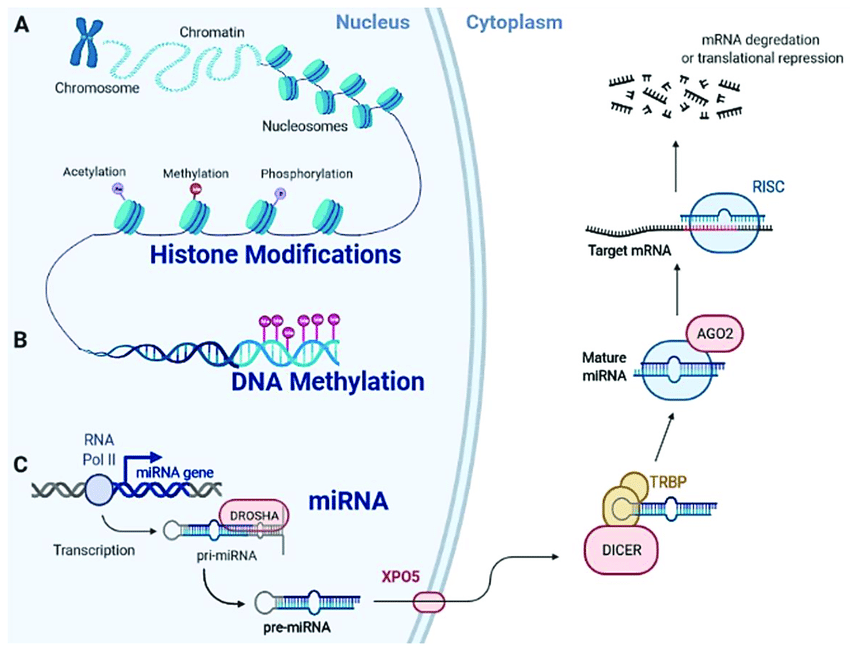
Cancer cells have a disrupted epigenome, characterized by abnormal DNA methylation patterns and histone modifications. These modifications silence genes that suppress tumors and activate oncogenes, promoting tumor growth and immune evasion. Additionally, the tumor microenvironment (TME), the surrounding cellular environment, is often immunosuppressive. Epigenetic alterations within immune cells like T lymphocytes in the TME can further suppress the immune system's attack on tumors.
Targeting Epigenetic Mechanisms to Enhance Immunotherapy
Two main drug classes target epigenetic modifications: DNA methyltransferase inhibitors (DNMTis) and histone deacetylase inhibitors (HDACis). DNMTis reverse DNA methylation, potentially reactivating silenced tumor suppressor genes and making tumors more susceptible to immune attack. HDACis modify histone acetylation patterns, promoting the expression of genes involved in antigen presentation and T cell activation, thereby stimulating the anti-tumor immune response. Researchers worldwide are actively investigating these drugs. Companies like Maxanim provide essential reagents, such as enzymes and antibodies, to support this critical research effort.
Combination Strategies: Improving Immunotherapy Efficacy
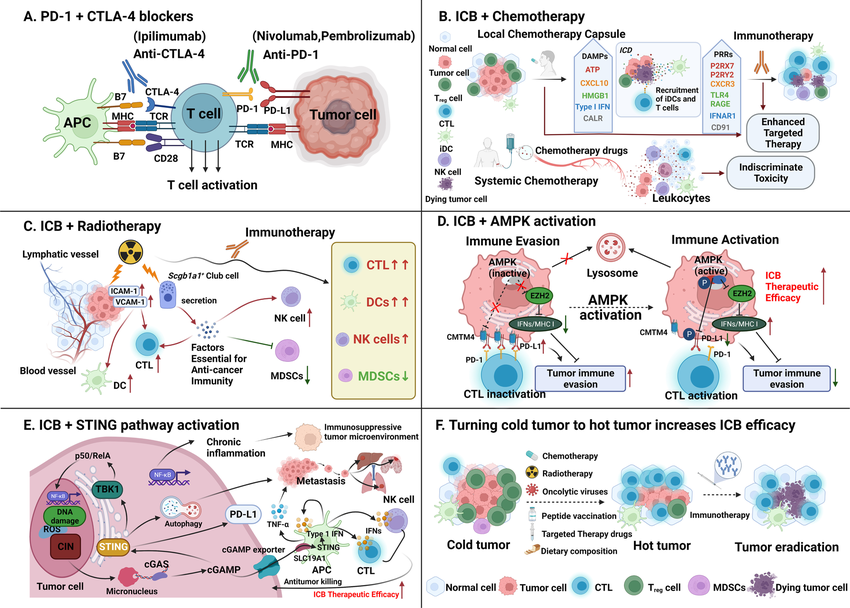
Combining epigenetic drugs with established immunotherapies, such as immune checkpoint inhibitors (ICIs), shows significant promise. ICIs block immunosuppressive pathways, but their effectiveness can be limited by an immunosuppressive TME. Epigenetic drugs can reprogram the TME, making tumor cells more vulnerable to T cell attack and strengthening the efficacy of ICIs.
Challenges and Future Directions
Despite promising pre-clinical and clinical data, challenges remain. Identifying the most effective epigenetic targets and tailoring therapies to specific tumor types and patient groups require further investigation. Additionally, understanding the complex interaction between epigenetic modifications and other aspects of the immune response is crucial for maximizing therapeutic benefit while minimizing side effects.
Conclusion
Epigenetic modifications play a critical role in both tumor development and immune evasion. Targeting these modifications with epigenetic drugs offers a powerful approach to enhance the effectiveness of cancer immunotherapy. Continued research holds the potential to refine combination strategies and personalize epigenetic therapies for improved patient outcomes.
Learn more in this video: