Chromatography is a fundamental analytical technique used to separate mixtures into their individual components. It finds widespread applications in various scientific fields, including biochemistry, pharmacology, environmental science, and forensics.
Separation Mechanism: Mobile and Stationary Phases
Chromatography relies on the interaction between two distinct phases:
- Mobile phase: Carries the sample mixture through the system. It can be a liquid (HPLC) or a gas (GC).
- Stationary phase: A solid matrix or coated packing material that interacts with the sample components through adsorption, partitioning, or size exclusion. These interactions determine the rate at which each component travels through the stationary phase, achieving separation.
The selection of the mobile and stationary phases is crucial for successful chromatography. Factors like polarity, size, and functionality of the components influence their interaction with the phases, ultimately affecting separation efficiency.
Chromatographic Techniques: Selection for Specific Separations
Chromatography offers a variety of techniques, each suited for specific sample types and separation requirements. Here are some of the most common methods:
- High-Performance Liquid Chromatography (HPLC): Utilizes a liquid mobile phase and a solid stationary phase, ideal for separating polar and ionic compounds.
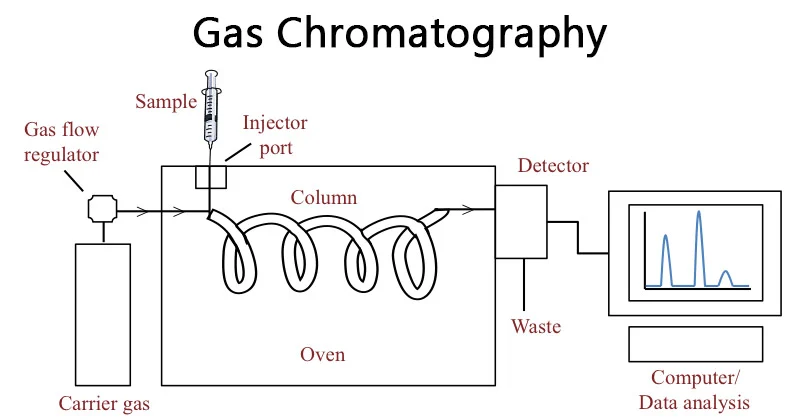
- Gas Chromatography (GC): Employs a gaseous mobile phase and a solid or liquid stationary phase, excelling at separating volatile and thermally stable compounds.
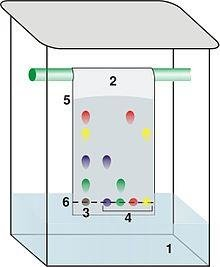
- Thin-Layer Chromatography (TLC): A simple and cost-effective technique using a stationary phase coated on a plate and a liquid mobile phase for rapid separations.
These techniques can be further combined with sophisticated detectors like mass spectrometers (MS) to not only separate components but also provide structural information about them.
Applications in Research: From Pharmaceuticals to Environmental Analysis
Chromatography's impact extends beyond analytical separations. Here are some key applications that showcase its versatility:
- Drug Discovery and Development: Plays a vital role in purifying potential drug candidates, analyzing metabolites, and ensuring drug purity.
- Environmental Monitoring: Aids in detecting and quantifying pollutants in air, water, and soil samples.
- Food Science and Safety: Scientists leverage chromatography to identify food adulterants, analyze food composition, and ensure food quality.
Conclusion: A Powerful Tool for Scientific Advancement
Chromatography, with its diverse techniques and continuous advancements, remains an essential tool for scientists. By harnessing the power of separation science, researchers can gain a deeper understanding of complex mixtures, driving innovation across numerous scientific fields.
For a deeper dive into HPLC, this video offers a clear explanation